The long-standing scientific focus of the Romer laboratory is molecular signaling in vascular injury, repair, and regeneration, with a special emphasis on post-translational modification by phosphorylation. Care has been taken over the past two decades to build a multidisciplinary team that incorporates expertise in the biochemistry, molecular biology, and mechanochemical signaling of cell adhesion, the cell biology of cytoskeletal organization, matrix biology, and regenerative medicine. A strong network of international and local collaboration and the outstanding efforts of students from the Biomedical Engineering, and Cell and Molecular Medicine programs at Johns Hopkins have supported a rich intellectually and ethnically diverse environment that has helped the lab to thrive.
Current and recent work includes the definition of mechanotransduction pathways in matrix assembly and cellular responses to microenvironmental geometry (separate grants funded by the Division of Materials Science Research, and by the Division of Chemical, Bioengineering, Environmental, and Transport Systems, both at the National Science Foundation), molecular mechanisms of NO dysregulation and vascular dysfunction in cardiovascular disease (funded by NHLBI, the American Heart Association, the MedImmune Corporation, a T32 postdoctoral fellowship from the JHU Children’s Center, Gilead Sciences Research Scholars Program in Pulmonary Arterial Hypertension, Pearl M. Stetler Research Fund for Women, and Pulmonary Hypertension Association), and microvascular tissue engineering and biomimetic vascular grafts (funded by the Posen Foundation, Kley Dom Biomimetics, and NICHD).
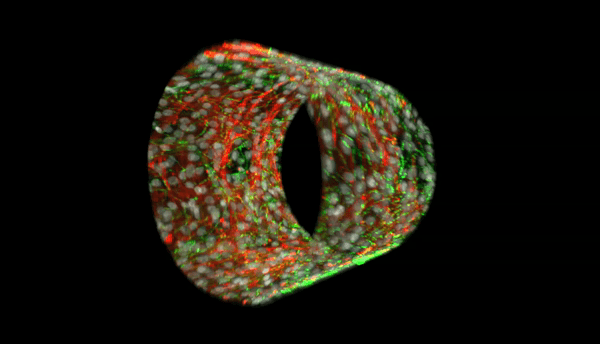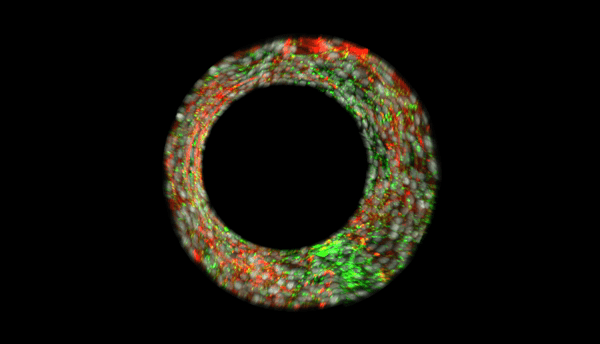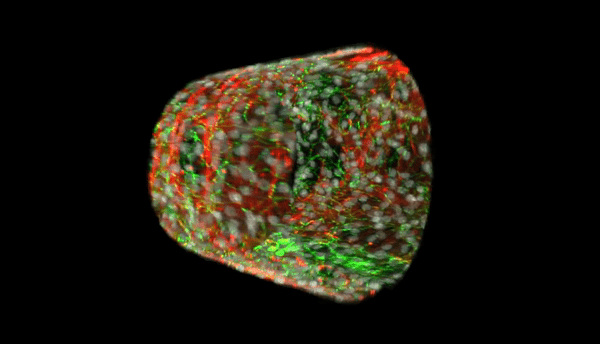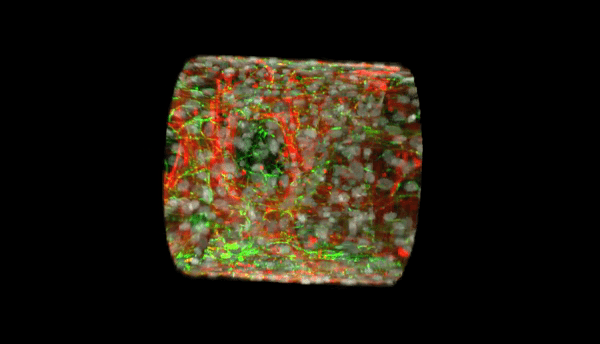
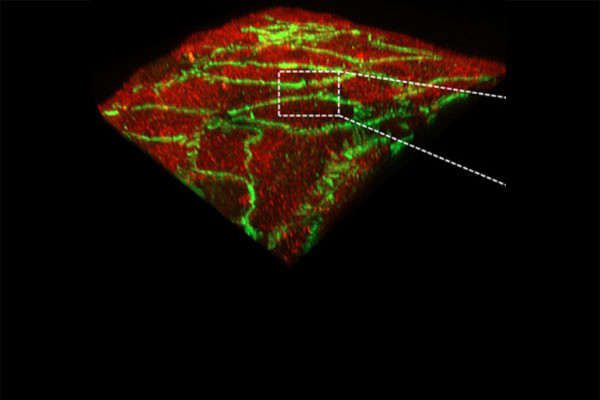
Our multi-level studies focus on vasculopathy in development, pulmonary hypertension, and atherogenesis, and range from transcriptional regulation, to the biochemistry of post-translational modification (phosphorylation, neddylation, and ubiquitination), and proteasomal degradation, to real time imaging of subcellular trafficking, to whole tissue and organism work with a novel transgenic mouse line with human HDAC2 expression that is specific to the vascular endothelium.

Lewis H. Romer is a Professor of Anesthesiology and Critical Care Medicine, Cell Biology, Biomedical Engineering and Pediatrics at the Johns Hopkins University School of Medicine. He has worked at the interface between cell signaling and the matrix microenvironment for the past three decades, making seminal contributions to our understanding of interplay between non-receptor tyrosine kinases and rho-family GTPases in cell adhesion, mechanochemical coupling in extracellular matrix assembly, and extracellular matrix guidance of vasculogenesis. Dr. Romer and his present and former trainees are advancing the fields of vascular tissue engineering, and biomechanics.

Meghan Bernier is a faculty member in the Division of Pediatric Anesthesiology and Critical Care Medicine and attends in the Pediatric Intensive Care Unit. Her research interests include clinical and translational studies in pediatric pulmonary hypertension with the ultimate goal of improving care and outcomes for these patients. In the lab, her work focuses on endothelial dysfunction in the pulmonary vasculature using cell culture techniques and animal models of pulmonary hypertension.

Xing Chen is currently working on biomimetic pulmonary microsystem at Johns Hopkins Department of Anesthesiology and Critical Care Medicine. He received his PhD degree in mechanical engineering from Chonnam National University, and then explored his diverse research interests in wireless sensing biomedical implant, implantable sensor packaging, and flexible sensor with application-specific integrated circuit interface for cardiovascular health at electrical engineering of University of British Columbia and University of Utah, respectively.

Qianru Jin works on mechanotransduction projects. Currently she is studying effects of cyclic stretch on fetal lung cells, in the context of nuclear mechanotransduction. She is interested in designing and creating a novel engineering platform to study geometry and mechanical effects on vascular and lung cells. She received her BS from Tsinghua University in Beijing, China in 2011, then began PhD study at the Johns Hopkins University, and joined the Romer lab in 2015.
In the rare occasion when she is not in Ross top floor or Maryland basement, she enjoys outdoors and reading.

Gayatri Pahapale is a graduate student in the Gracias lab and is co-advised by Dr. Romer. Her work is aimed at building a platform to study the effect of physical constraints on a cell using gels, which mimic the cell matrix very closely. She received her Bachelor's degree in Chemical Technology (B. Tech) from the Institute of Chemical Technology in Mumbai, India in 2016, after which she began graduate study at Johns Hopkins.

Michal Levine Millrod earned her PhD from the Immunology Program in the Department of Molecular Biology and Genetics at the Johns Hopkins School of Medicine. Her thesis work was conducted in the Institute for Cell Engineering and focused on the differentiation of human pluripotent stem cells (hPSC) to hematopoietic and cardiac lineages. She began her postdoctoral studies in the Romer Lab in 2018, where she is interested in using hPSC to develop personalized medicine approaches to studying and treating pediatric pulmonary hypertension.
Hyun Woo Sung
(* denotes equal contribution)
Qianru Jin, Anil Bhatta, Jayson V. Pagaduan, Xing Chen, Hoku West-Foyle, Jiayu Liu, Annie Hou, Dan Berkowitz, Scot Kuo, Frederic B. Askin, Thao D. Nguyen, David H. Gracias, and Lewis H. Romer. Biomimetic human small muscular pulmonary arteries. Science Advances, In Press, January 3, 2020; Sci Adv. 2020 Mar 25;6(13):eaaz2598. doi: 10.1126/sciadv.aaz2598. eCollection 2020 Mar.
PMID: 32232160
Emilio Bachtiar, Ozan Erol, Michal Millrod, Runhan Tao, David Gracias, Lew Romer, Sung Hoon Kang.
3D printing and characterizations of a soft and biostable elastomer with high flexibility and strength for biomedical applications, J Mech Behav Biomed Mater. 2020 Apr;104:103649. doi: 10.1016/j.jmbbm.2020.103649. Epub 2020 Jan 23.
Pahapale, G, Gao, S, Romer, L,* Gracias, D.* Hierarchically curved gelatin for 3D biomimetic cell culture. ACS Applied Bio Materials, Manuscript Status: Accepted, 6-Nov-2019. ACS Appl. Bio Mater. 2019, 2, 12, 6004-6011
ublication Date: November 6, 2019
https://doi.org/10.1021/acsabm.9b00916
PMID: 32174407
Bernier, M, Romer, L, Bembea, M. Spectrum of Current Management of Pediatric Pulmonary Hypertensive Crisis. Critical Care Explorations, Accepted, 11-Jul-2019. Published: 2019 Aug 9;1(8):e0037. doi: 10.1097/CCE.0000000000000037. eCollection 2019 Aug.
PMID: 32166278
Jiang, Z, Erol, O, Chatterjee, D, Xu, W, Hibino, N, Romer, L, Kang, SH, Gracias, D. Direct Ink Writing of Polytetrafluoroethylene (PTFE) with Tunable Mechanical Properties. ACS Applied Materials & Interfaces. Accepted, 10-Jul-2019. Published: 2019 Aug 7;11(31):28289-28295. doi: 10.1021/acsami.9b07279. Epub 2019 Jul 23.
PMID: 31291075
Liu, J, Erol, O, Pantula, A, Liu, W, Jiang,Z, Kobayashi, K, Chatterjee, D, Hibino, N, Romer, LH, Kang, SH,
Nguyen, TD, and Gracias, D. Dual-gel 4D Printing of Bioinspired Tubes. ACS Applied Materials & Interfaces, 2019 Feb 27;11(8):8492-8498. doi: 10.1021/acsami.8b17218. Epub 2019 Feb 12. PMID: 30694051
Rojas, T, Cheng, W, Gao, Z, Liu, X, Wang, Y, Malla, AP, Chin, AC, Romer, LH, Snyder, SH, and Fu, C. Inositol hexakisphosphate kinase 3 promotes focal adhesion turnover via interactions with dynein intermediate chain 2.
PNAS, 2019 Feb 19;116(8):3278-3287. doi: 10.1073/pnas.1817001116. Epub 2019 Feb 4. PMID: 30718399
Pandey, D, Nomura, Y, Rossberg, MC, Hori, D, Bhatta, A, Keceli, G, Leucker, T, Santhanam, L, Shimoda, LA, Berkowitz, D*, Romer, L*. Hypoxia Triggers SENP1 Modulation of Kruppel-Like Factor 15 and Transcriptional Regulation of Arginase 2 in Pulmonary Endothelium. Arterioscler Thromb Vasc Biol.,38:913-926, April, 2018. DOI: 10.1161/ATVBAHA.117.310660.
Pagaduan, JV, Bhatta, A, Romer, LH*, Gracias, DH*. 3D Hybrid Small Scale Devices, Small, February, 2018 May 10:e1702497. doi: 10.1002/smll.201702497. [Epub ahead of print]. PMID: 29749014.
Bernier, ML, Jacob, AI, Collaco, JM, McGrath-Morrow, SL, Romer, LH, Unegbu, CC. Perioperative Events in Children with Pulmonary Hypertension Undergoing Non-cardiac Procedures. Pulmonary Circulation. Accepted, 2017.
Jin, Q., Pahapale, G., Bhatta, A., Romer, L.*, and Gracias, DH*. MIcro Three-dimensional Squeezers (MiTS) for Investigating Single Cell Behavior under Confinement. MicroTAS Conference Proceedings, 2017.
Chen, X, Pagaduan, JV, Bhatta, A., Gracias, DH*, and Lewis Romer*. A Microfluidic Device For Trapping And Dynamic Interrogation Of Arterioles. MicroTAS Conference Proceedings, 2017.
Barreto Ortiz, SB, Hori, D, Nomura, Y, Yun, X, Jiang, H, Yong, H, Chen, J, Paek, S, Pandey, D, Sikka, G, Bhatta, A, Gillard, A, Steppan, J, Kim, J, Adachi, H, Barodka, V, Romer, L, An, S, Shimoda, L, Santhanam, L, and Berkowitz, DE. Opsin 3 and 4 Mediate Light-Induced Pulmonary Vasorelaxation that is Potentiated by G-Protein Receptor Kinase 2 Inhibition. Am J Physiol Lung Cell Mol Physiol. 2017 Sep 7:ajplung.00091.2017. doi: 10.1152/ajplung.00091.2017. PMID: 28882814.
Krishnan, U, Feinstein, JA, Adatia, I, Austin, E, Mullen, M, Hopper, R, Hanna, B, Romer, L, Keller, R, Fineman, J, Steinhorn, R, Kinsella, J, Ivy, DD, Rosenzweig, EB, Raj, U, Humpl, T, Abman, SH, for the Pediatric Pulmonary Hypertension Network (PPHNet). Evaluation and Management of Pulmonary Hypertension in Children with Bronchopulmonary Dysplasia, J. Pediatrics, 2017 Sep;188:24-34.e1. doi: 10.1016/j.jpeds.2017.05.029. Epub 2017 Jun 20. PMID: 28645441.
Unegbu, C, Noje, C, Coulson, J, Segal, J, Romer, L. State of the Art Review: Pulmonary Hypertension Therapy and a Systematic Review on Efficacy and Safety of PDE-5 Inhibitors. Pediatrics. February 24, 2017.
Leucker, T, Nomura, Y, Kim, JH, Bhatta, A, Wang, V, Wecker, A, Jandu, S, Santhanam, L, Berkowitz, D, Romer, L, Pandey, D. Histone deacetylase 6 inhibition provides protection against oxidized LDL-induced endothelial injury through upregulation of cystathionine -lyase. Epublication, Am J Physiol Heart Circ Physiol, February 10, 2017. doi:10.1152/ajpheart.00724.2016.
Kwag HR, Serbo JV, Korangath P, Sukumar S, Romer LH*, Gracias DH*. A Self-Folding Hydrogel In Vitro Model for Ductal Carcinoma. Tissue Eng Part C Methods. 2016 Apr;22(4):398-407. doi: 10.1089/ten.TEC.2015.0442. Epub 2016 Mar 16. PubMed PMID: 26831041; PubMed Central PMCID: PMC4827285.
Serbo JV, Kuo S, Lewis S, Lehmann M, Li J, Gracias DH, Romer LH. Patterning of Fibroblast and Matrix Anisotropy within 3D Confinement is Driven by the Cytoskeleton. Adv Healthcare Mater. 2016 Jan 7;5(1):146-58. doi: 10.1002/adhm.201500030. Epub 2015 Jun 1. PubMed PMID: 26033825.
Maxwell BG, Nies MK, Ajuba-Iwuji CC, Coulson JD, Romer LH. Trends in Hospitalization for Pediatric Pulmonary Hypertension. Pediatrics. 2015 Aug;136(2):241-50. doi: 10.1542/peds.2014-3834. Epub 2015 Jul 6. PubMed PMID: 26148956.
Deepesh Pandey, Daijiro Hori, Jae Hyung Kim, Dan E. Berkowitz, L Romer. Neddylation Promotes Endothelial Dysfunction: A Role for HDAC2. Journal of Molecular and Cellular Cardiology. Apr;81:18-22.Available On Line February 2, 2015. doi: 10.1016/j.yjmcc.2015.01.019.
Soucy PA, Hoh M, Heinz W, Hoh J, Romer L. Oriented matrix promotes directional tubulogenesis. Acta Biomaterialia, 2015 Jan 1;11:264-73. doi: 10.1016/j.actbio.2014.08.037. Epub 2014 Sep 8. PMID:25219769.
Chang F, Lemmon CA, Nilaratanakul V, Rotter V, Romer L. Endothelial Matrix Assembly During Capillary Morphogenesis: Insights from Chimeric TagRFP-Fibronectin Matrix. J Histochem Cytochem. 2014 Jul 25. pii: 0022155414547419. [Epub ahead of print] No abstract available. PMID: 25063001.
Pandey D, Bhunia A, Oh YJ, Chang F, Bergman Y, Kim JH, Serbo J, Boronina TN, Cole RN, Van Eyk J, Remaley AT, Berkowitz DE*, Romer LH*. OxLDL Triggers Retrograde Translocation of Arginase2 in Aortic Endothelial Cells via ROCK and Mitochondrial Processing Peptidase. Circ Res. 2014 Aug 1;115(4):450-9. doi: 10.1161/CIRCRESAHA.115.304262. Epub 2014 Jun 5. PMID: 24903103.
Pandey D, Sikka G, Bergman Y, Kim JH, Ryoo S, Romer L*, Berkowitz D*. Transcriptional regulation of endothelial arginase 2 by histone deacetylase 2. Arterioscler Thromb Vasc Biol. 2014 Jul;34(7):1556-66. doi: 10.1161/ATVBAHA.114.303685. Epub 2014 May 15. PMID: 24833798.
Teo B, Wong ST, Lim CK, Kung T, Yap CH, Ramgopal Y, Romer L, Yim E. Nanotopography Modulates Mechanotransduction of Stem Cell and Induces Differentiation Through Focal Adhesion Kinase. ACS Nano,7 (6):4785–4798, 2013; doi: 10.1021/nn304966z; Epub May 13, 2013.
Brown AT, Gillespie JV, Miquel-Verges F, Holmes K, Ravekes W, Spevak P, Brady K, Easley RB, Golden WC, McNamara L, Veltri MA, Lehmann CU, McMillan KN, Schwartz JM, Romer LH. Inhaled epoprostenol therapy for pulmonary hypertension: Improves oxygenation index more consistently in neonates than in older children. Pulm Circ. 2012 Jul 6. doi: 10.1002/ppul.22609.
Collaco, JM, Romer, LH, Stuart, BD, Coulson, JD, Everett, AD, Lawson, EE, Brenner, JI, Brown, AT, Nies, MK, Sekar, P, Nogee, LM, McGrath-Morrow, SA, Frontiers in Pulmonary Hypertension in Infants and Children with Bronchopulmonary Dysplasia. Pediatric Pulmonology. 2012 Nov;47(11):1042-53. doi: 10.1002/ppul.22609. Epub 2012 Jul 6.
Chang, F, Lemmon, C, Lietha, D, Eck, M, and Romer, L. Tyrosine Phosphorylation of Rac1: A Role in Regulation of Cell Spreading. PLoS ONE, 6 (12): e28587, Dec 6, 2011.
Lemmon CA, Romer LH. A Predictive Model of Cell Traction Forces Based on Cell Geometry. Featured Biophysical Letter in Biophysical Journal, 2010; Nov;99:L78-L80.
Ryoo S, Bhunia AK, Chang F, Shoukas A, Berkowitz DB*, Romer LH.* OxLDL-Dependent Activation of Arginase is Dependent on the LOX-1 Receptor and Downstream RhoA Signaling. In press, Atherosclerosis, 2010. Epub ahead of print, Nov 4, 2010. In print, 2011;214(2):279-87.
Soucy PA, Werbin J, Heinz W, Hoh J, Romer,LH. Microelastic properties of lung cell-derived extracellular matrix. Acta Biomaterialia, epub July 23, 2010; in print 2011;7: 96–105.
Lemmon CA, Chen CS, Romer LH. Cell Traction Forces Direct Fibronectin Matrix. Biophysical Journal, 2009;96: 729–738.
Soucy PA, Romer LH. Endothelial Cell Adhesion, Signaling, and Morphogenesis in Fibroblast-Derived Matrix. Matrix Biology. Epub ahead of print, April 17, 2009.
Lemmon CA, Romer LH. Measuring Patterns, Regulation, and Biologic Consequences of Cellular Traction Forces. Gravitational and Space Biology. 2007;June; 20(2):19-29
Usatyuk P, Romer LH, Zhan S, Garcia JGN, Natarajan V. Regulation of Hyperoxia-induced NADPH Oxidase Activation in Human Lung Endothelial Cells by the Actin Cytoskeleton and Cortactin. J Biol Chem. 2007;282(32):23284-95.
Werbin J, Heinz W, Romer L, Hoh J. Micropatterns of an Extracellular Matrix Protein with Defined Information Content. Langmuir. Epub Sep 22 2007; 2007;Oct 23;23(22):10883-6.
Pirone DM, Liu W, Raghavan L, Romer LH, Chen CS. Focal adhesion kinase controls shape-dependent endothelial cell proliferation. J Cell Biology. 2006;174:277-88.
Shin S, Maneesh P-S, Lee J-S, Romer LH, Kim KS. Focal Adhesion Kinase is Involved in Group B Streptococcal Invasion of Human Brain Microvascular Endothelial Cells. Molecular Pathogenesis. 2006;41:168-73.
Ryoo S, Lemmon CA, White AR, Soucy KG, Nyhan D, Shoukas A, Romer LH*, Berkowitz DE*. Ox-LDL-Dependent Endothelial Arginase II Activation Contributes to Impaired NO Signaling. Circulation Research. 2006;99:951-960.
Romer LH, Birukov KG, Garcia JGN. The Focal Adhesion: Paradigm for a Signaling Nexus. Circulation Research, 2006;98;606-616.
Chang F, Lemmon CA, Park D, Romer LH. FAK Potentiates Rac1 Activation and Localization to Matrix Adhesion Sites: A Role for betaPIX. Molecular Biology of the Cell. 2007;Jan;18(1):253-64, Epub 2006 Nov 8.
Wadgaonkar R, Dudek SA, Zaiman AL, Linz-Mcgillem L, Verin AD, Nurmukhambetova S, Romer LH, Garcia JGN. Intracellular Interaction of Myosin Light Chain Kinase With Macrophage Migration Inhibition Factor (MIF) In Endothelium. J Cell Biochem. 2005; Apr 18, (Epub ahead of print), 2005.
Lemmon CA, Sniadecki N, Alom Ruiz S, Tan J, Romer LH*, Chen CS.* Shear force at the cell-matrix interface: enhanced analysis for microfabricated post array detectors. Mechanics and Chemistry of Biosystems, 2005;2:1-16.
Rhoads M, Argenzio R, Chen W, Sheth P, Romer LH. L-arginine stimulates intestinal cell migration through a nitric oxide- and polyamine-dependent mechanism. Gut, 2004;53:514-22.
Petrache I, Birukov K, Zaiman AL, Crow MT, Deng H, Wadgaonkar R, Romer LH, Garcia JGN. Caspase-dependent cleavage of myosin light chain kinase (MLCK) is involved in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. FASEB J. 2003;17:407-416.
Young BA, Sui X, Kiser TD, Hyun SW, Wang P, Sakarya S, Angelini DJ, Schaphorst KL, Hasday JD, Cross AS, Romer LH, Passaniti A, Goldblum SE. Protein Tyrosine Phosphatase Activity Regulates Endothelial Cell-Cell Interactions, the Paracellular Pathway and Capillary Tube Stability. Am J Physiol Lung Cell Mol Physiol. 2003;285: L63–L75.
Katz BT, Romer LH, Miyamoto S, Volberg T, Matsumoto K, Cukierman E, Geiger B, Yamada K. Targeting Membrane-Localized Focal Adhesion Kinase to Focal Adhesions: Roles of Tyrosine Phosphorylation and Src Family Kinases. J Biol Chem. 2003; 278:29115–29120.
Rajfur Z, Roy P, Otey C, Romer LH, Jacobson KA. Chromophore-assisted laser inactivation (CALI) of EGFP fusion proteins: probing the connection between the stress fiber and the focal adhesion. Publication pending, Nature Cell Biology, 2002;4:286-293.
Volberg T *, Romer LH*, Zamir E, Geiger B. pp60c-src and related tyrosine kinases: a role in the assembly and reorganization of matrix adhesions. J Cell Science, 2001;114:2279-2289.
Gilmore A, Romer LH. Inhibition of FAK Signalling in Focal Adhesions Decreases Cell Motility and Proliferation. Molecular Biology of the Cell. 1996;7:1209-1224.
Romer LH, McLean NV, Horng-Chin Y, Daise M, Sun J, DeLisser HM. TNFa and gamma interferon induce redistribution of PECAM-1 on human endothelial cells. J Immunology. 1995; 154:6582-6592.
Romer LH, McLean NV, Turner CT, Burridge K. Tyrosine kinase activity, cytoskeletal organization, and motility in human vascular endothelial cells. MolecularBiology of the Cell. 1994;5:349-361.
Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: A role in cytoskeletal assembly. J Cell Biology. 1992;119:893-904.
Burridge K, Petch LA, Romer LH. Signals from focal adhesions. Current Biology, 1992;2:537-539.
Albelda SE, Oliver PD, Romer LH, Buck CA. Endocam: A novel cell-cell adhesion molecule. J Cell Biology. 1990;110: 1227-1237.
Post Analysis

This page contains MATLAB .m files that analyze data collected on microfabricated post array detectors (mPADs). These programs are free to download, but we ask that if you use them you site the following paper:
Lemmon CA, Sniadecki NJ, Alom Ruiz S, Tan JL, Romer LH, and Chen CS. “Shear force at the cell-matrix interface: enhanced analysis for microfabricated post array detectors”, Mechanics and Chemistry of Biosystems, 2005.
PostAnalysis 1.41
PostAnalysis 1.41 consists of a series of m-files that analyze images acquired from the microfabricated post array detector (mPAD) system. M-files are contained in a zip file, and are intended for use with Matlab version 6.5.1 and later.
Download PostAnalysis 1.41
(Right-click and choose “Save Target As”)
Installation:
After downloading the zip file, extract the m-files with WinZip. The folder containing the m-files must be added to the working path of Matlab. After starting Matlab, click on the ‘File’ menu tab and select ‘Set Path’. Then click on ‘Add with Subfolders’. Select the folder containing the m-files and click ‘ok’. Verify that the file name has been added and click ‘save’. Then click ‘ok’.
Running the Program:
PostAnalysis 1.41 must be run from the folder containing the images to be analyzed. First, switch the ‘Current Directory’ panel (on the left side of the Matlab screen in Matlab 6.5.1; at the top of the screen in Matlab 7) to the directory containing images to be analyzed. (Use the ‘…’ button to find the directory). In order for the program to run properly, the folder must contain three 12-bit TIF files with the suffix ‘Top.tif’, ‘Bottom.tif’, and ‘Actin.tif’ and the same prefix. You do not need to select these files; just verify that they are present in the current directory. After switching to the proper directory, type ‘analyze_posts_LT2’ (without the quotes) at the Matlab command prompt and press ‘enter’. A graphics window will open and will prompt for all needed inputs. Follow the instructions as directed. Some processes, such as rotation and cropping, can take 10-20 seconds to complete.
Troubleshooting/FAQ:
When I start the code, I receive an error saying that the variable ‘dir_names’ is unknown. The program cannot find the appropriate image files in the current directory. Verify that there are images with the appropriate suffixes in the current directory (see above).
Nothing seems to happen when I try to click on rotation points, drag the crop box, or select collapsed posts. You must click ‘ok’ on the textbox before the program will allow you to begin the operation.
I’ve selected collapsed posts, but can’t continue in the program. You must hit ‘enter’ with the crosshairs located over the image to continue.
I selected collapsed posts, but can’t tell which ones I’ve selected. Marked posts will not be labeled with yellow ’s until after you have hit ‘enter’ to continue (see above). You will have a chance to clear them and select again if you make mistakes.
I can’t get the fibronectin or vinculin analysis to run. At this point in time, modules for fibronectin and vinculin analysis have not been completed.
The program seems to have frozen. If you encounter a problem, you can use ‘ctrl-c’ to stop execution of the program.
Graphics Requirement: PostAnalysis 1.41 requires that screen resolution be set at least 1024 x 768 pixels.
Questions/Comments/Concerns:
Please contact Lew Romer ([email protected]),
Chris Chen ([email protected]), or
Chris Lemmon ([email protected]) with questions or comments about the program and/or mPAD system.
Please direct technical questions related to the code functionality to Chris Lemmon ([email protected]).